Biography
Stefan Hervø-Hansen is a JSPS postdoctoral researcher at Osaka University in the Molecular-Aggregate Chemical Engineering group. His research interests include molecular simulations, free-energy calculation methods, and rational macromolecular design. During Stefan’s MSc and PhD his research mainly focused on the importance of pH and co-solvent effects on the perturbation of molecular equilibria in a high variety of different chemical systems.
Having been awarded the JSPS fellowship for his proposal on the topic “Protein Solubility and Aggregation in Solution”, Stefan now focuses on providing an atomistic description of solvation of macromolecules in solution. This step is one in many towards the ultimate goal for: “Combining Machine Learning and Physics-based Simulations for Smart Design of Materials”
- Molecular Simulations
- Free Energy Calculations
- Protein design
PhD in Theoretical Chemistry, 2021
Lund University
MSc in Protein Chemistry, 2017
University of Copenhagen
BSc in Biochemistry, 2015
University of Copenhagen
Experience
Conducted research on the usage of free energy calculation for rational modification of molecular matter.
Conducted public speaking at high schools in Japan on topics such as research, life in academics, and usage of English.
Research funded by the Japan Society for Promotion of Science (JSPS), with the host institution being Osaka University.
Supervisor: Nobuyuki Matubayasi
Conduced research on the thermodynamic solvation of biomolecules in solution using insilico methods
Teaching conducted:
- Statistical Thermodynamics and Molecular Simulations
- Python Programming for Chemists
Supervisor: Mikael Lund
Awards & Grants
Recent Posts
Featured Publications
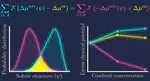
Solvent environment may significantly affect the equilibria involving flexible solute species, such as proteins and polymers. In the present work, a computation scheme is formulated for the change in the excess chemical potential of a flexible solute molecule upon variation of the solvent condition. The formulation adopts the scheme of error minimization in parallel to the method of Bennett acceptance ratio, and an exact expression is presented that provides the change in the excess chemical potential from solvation free energies computed in two solvent conditions of interest. The formulated scheme is applied to n-hexanol as the solute in water and n-octanol as the two solvent systems and to an oligomer of ethylene glycol as the solute in water with urea or NaCl added as a cosolvent. It is demonstrated that the change in the excess chemical potential of the solute due to the variation of the solvent condition (composition) can be obtained from the solvation free energies calculated over ∼10 to ∼102 solute configurations, without referring to any intermediate states between the two solvent conditions concerned. The connection to the solvent-condition dependence of the solute structure is further discussed for the systems of the ethylene-glycol oligomer, and the role is addressed for the probability distribution functions of the cosolvent-induced changes in the solvation free energies.
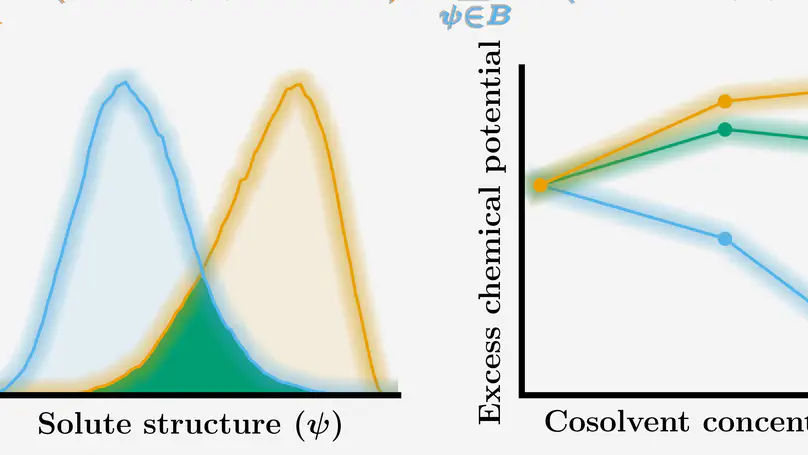
The roles of cations and anions are different in the perturbation on solvation, and thus, the analyses of the separated contributions from cations and anions are useful to establish molecular pictures of ion-specific effects. In this work, we investigate the effects of cations, anions, and water separately in the solvation of n-alcohols and n-alkanes by free-energy decomposition. By utilising energy-representation theory of solvation, we address the contributions arising from the direct solute–solvent interactions and the excluded-volume effects. It is found that the change in solvation of n-alcohols and n-alkanes upon addition of salt depends primarily on the anion species. The direct interaction between the anion and solute is in agreement with the Setschenow coefficient in terms of the ranking of salting-in and salting-out for n-alkanes, which corresponds to the extent of accumulation of the anion on the solute surface. For each of the n-alcohols and n-alkanes examined, the excluded-volume component in the Setschenow coefficient is well correlated to the (total) Setschenow coefficient when the salt effects are concerned. The ranking of the excluded-volume component in the variation of the salt species is parallel to the water contribution, which is correlated further to the change in the water density upon the addition of the salt.
Salts affect the solvation thermodynamics of molecules of all sizes; the Hofmeister series is a prime example in which different ions lead to salting-in or salting-out of aqueous proteins. Early work of Tanford led to the discovery that the solvation of molecular surface motifs is proportional to the solvent accessible surface area (SASA), and later studies have shown that the proportionality constant varies with the salt concentration and type. Using multiscale computer simulations combined with vapor-pressure osmometry on caffeine-salt solutions, we reveal that this SASA description captures a rich set of molecular driving forces in tertiary solutions at changing solute and osmolyte concentrations. Central to the theoretical work is a new potential energy function that depends on the instantaneous surface area, salt type, and concentration. Used in, e.g., Monte Carlo simulations, this allows for a highly efficient exploration of many-body interactions and the resulting thermodynamics at elevated solute and salt concentrations.
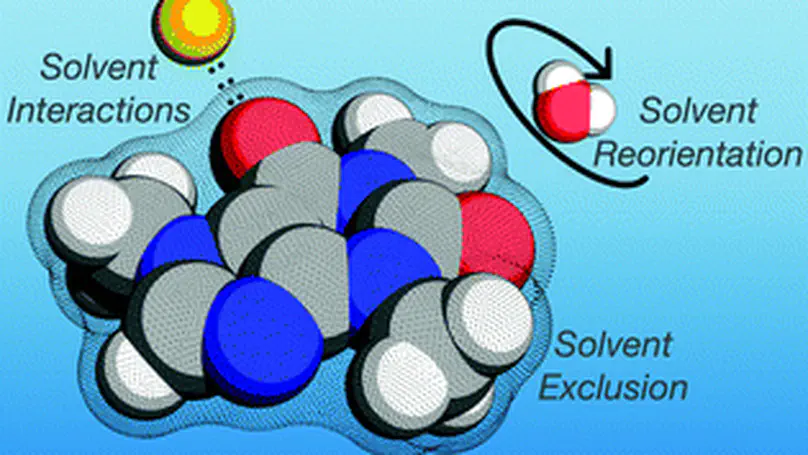
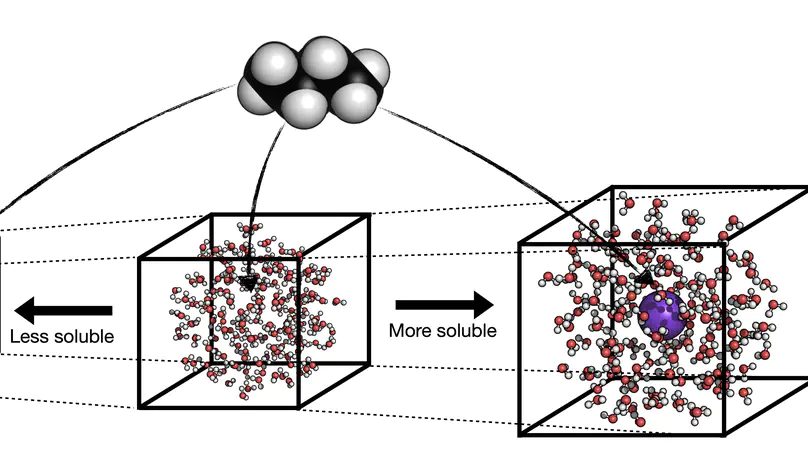
The contributions from anions and cations from salt are inseparable in their perturbation of molecular systems by experimental and computational methods, rendering it difficult to dissect the effects exerted by the anions and cations individually. Here we investigate the solvation of a small molecule, caffeine, and its perturbation by monovalent salts from various parts of the Hofmeister series. Using molecular dynamics and the energy-representation theory of solvation, we estimate the solvation free energy of caffeine and decompose it into the contributions from anions, cations, and water. We also decompose the contributions arising from the solute–solvent and solute-ions interactions and that from excluded volume, enabling us to pin-point the mechanism of salt. Anions and cations revealed high contrast in their perturbation of caffeine solvation, with the cations salting-in caffeine via binding to the polar ketone groups, while the anions were found to be salting-out via perturbations of water. In agreement with previous findings, the perturbation by salt is mostly anion dependent, with the magnitude of the excluded-volume effect found to be the governing mechanism. The free-energy decomposition as conducted in the present work can be useful to understand ion-specific effects and the associated Hofmeister series.
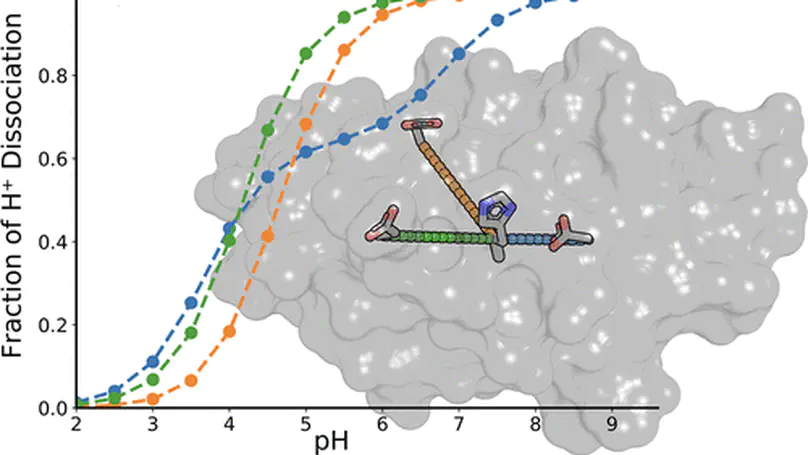
Electrostatic forces are important for protein folding and are favored targets of protein engineering. However, interactions between charged residues are difficult to study because of the complex network of interactions found in most proteins. We have designed a purposely simple system to investigate this problem by systematically introducing individual and pairs of charged and titratable residues in a protein otherwise free of such residues. We used constant pH molecular dynamics simulations, NMR spectroscopy, and thermodynamic double mutant cycles to probe the structure and energetics of the interaction between the charged residues. We found that the partial burial of surface charges contributes to a shift in pKa value, causing an aspartate to titrate in the neutral pH range. Additionally, the interaction between pairs of residues was found to be highly context dependent, with some pairs having no apparent preferential interaction, while other pairs would engage in coupled titration forming a highly stabilized salt bridge. We find good agreement between experiments and simulations and use the simulations to rationalize our observations and to provide a detailed mechanistic understanding of the electrostatic interactions.
Recent Publications
Contact
- stefan@cheng.es.osaka-u.ac.jp
- +81 080-8544-4852
- 1-3 Machikaneyama-cho, Toyonaka-shi, Osaka 560-8531
- Enter the building and take the stairs to Floor 2. Walk stright to the end of the corridor.
- Monday-Friday 10:00 to 17:00